Amelioration of Metal-Induced Cellular Stress by α-Lipoic Acid and Dihydrolipoic Acid through Antioxidative Effects in PC12 Cells and Caco-2 Cells
Abstract
1. Introduction
2. Materials and Methods
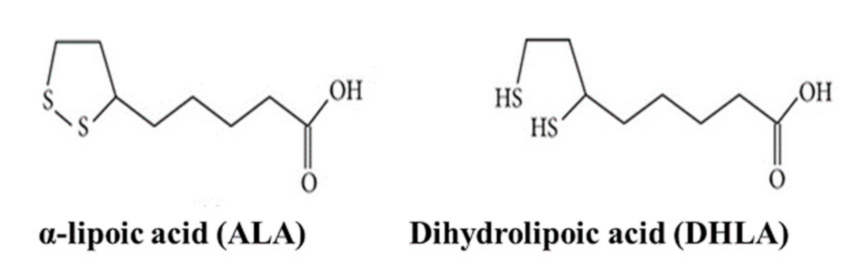
2.1. Materials
2.2. Cell Culture
2.3. Cell Viability
2.4. Lactate Dehydrogenase (LDH) Activity Assay
2.5. Measurement of GSH Levels
2.6. Isolation of Genomic DNA and Agarose Gel Electrophoresis
2.7. Western Blot Analysis for Determination of Protein Expression
2.8. Statistical Analysis
3. Results
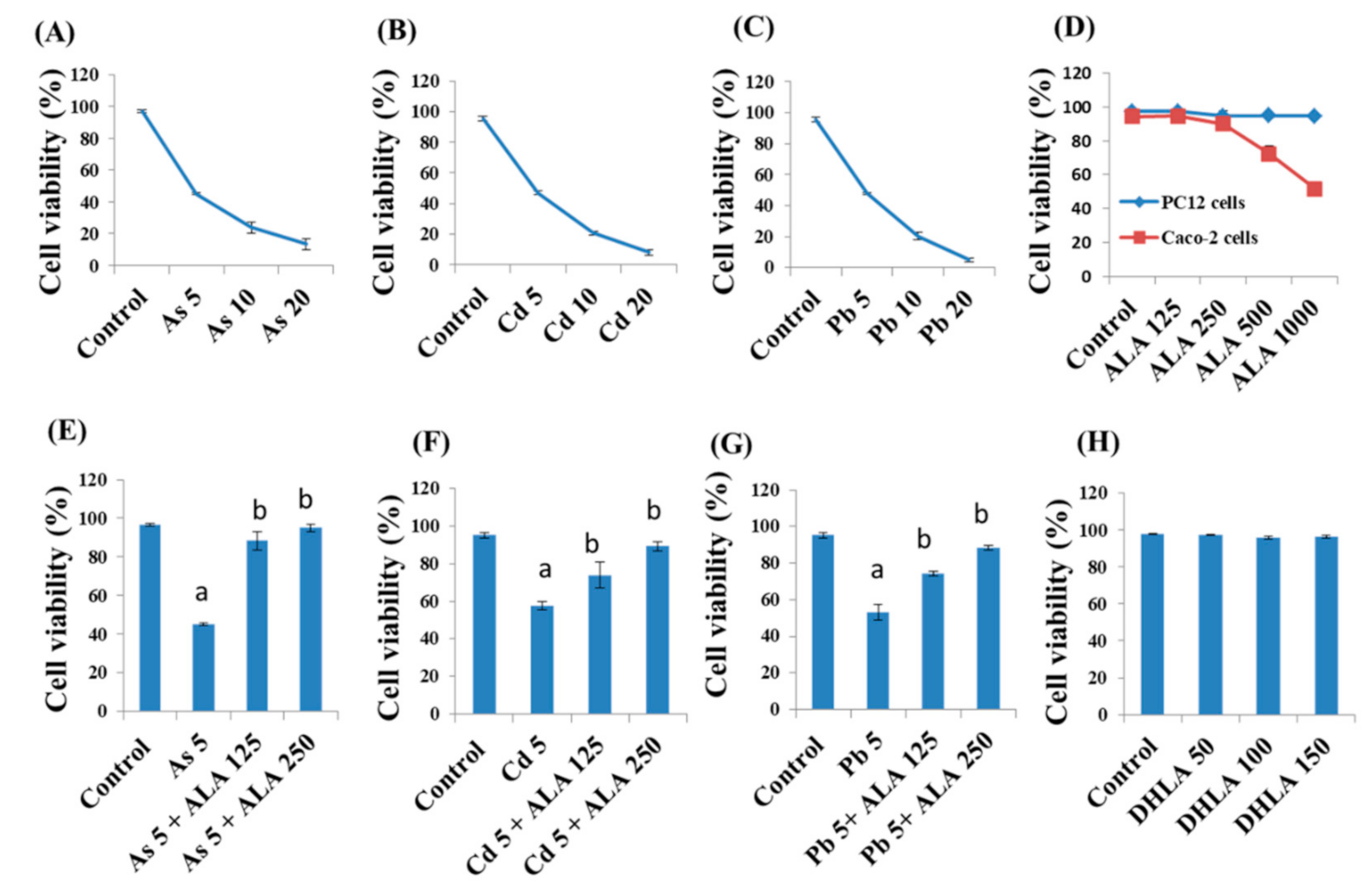
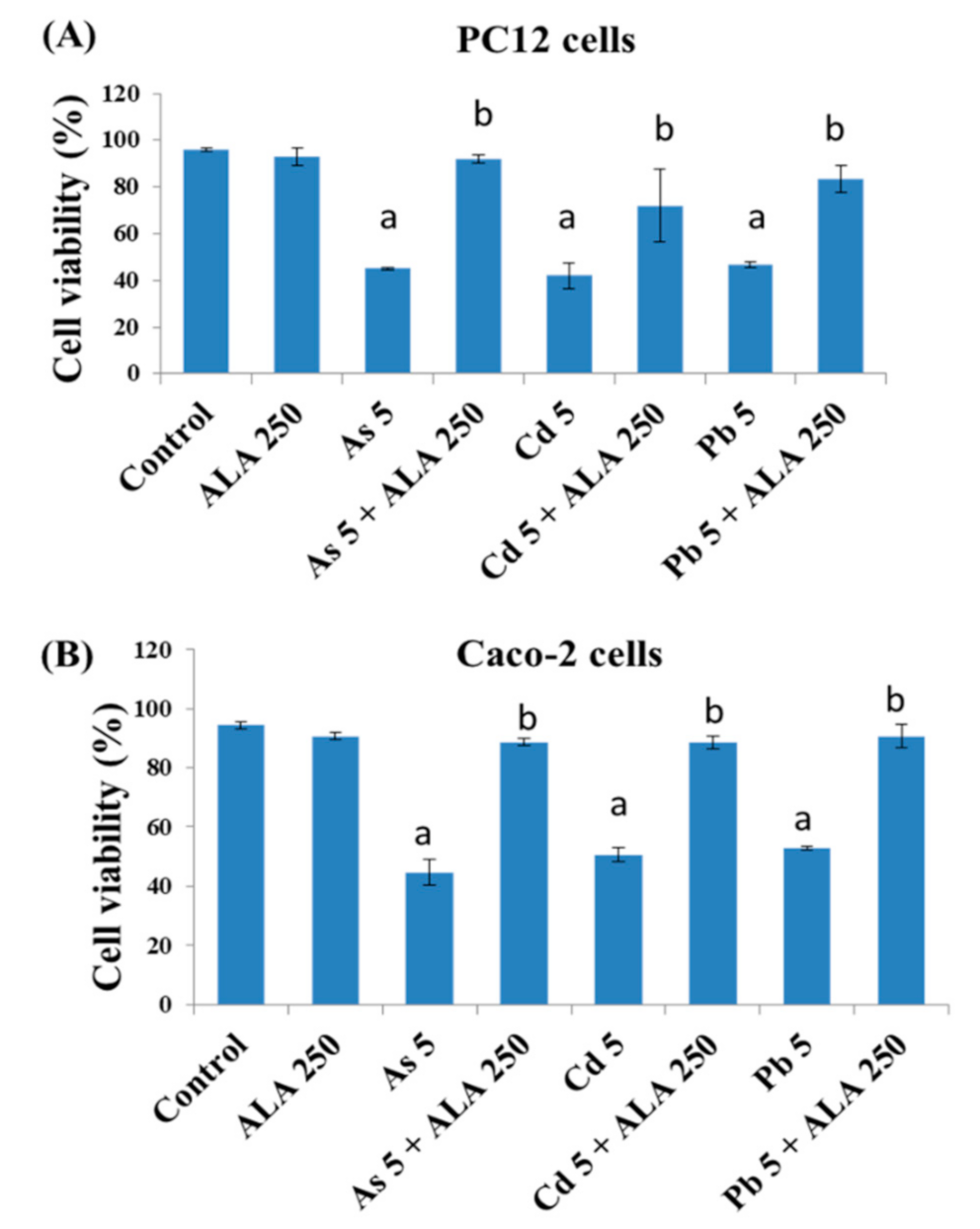
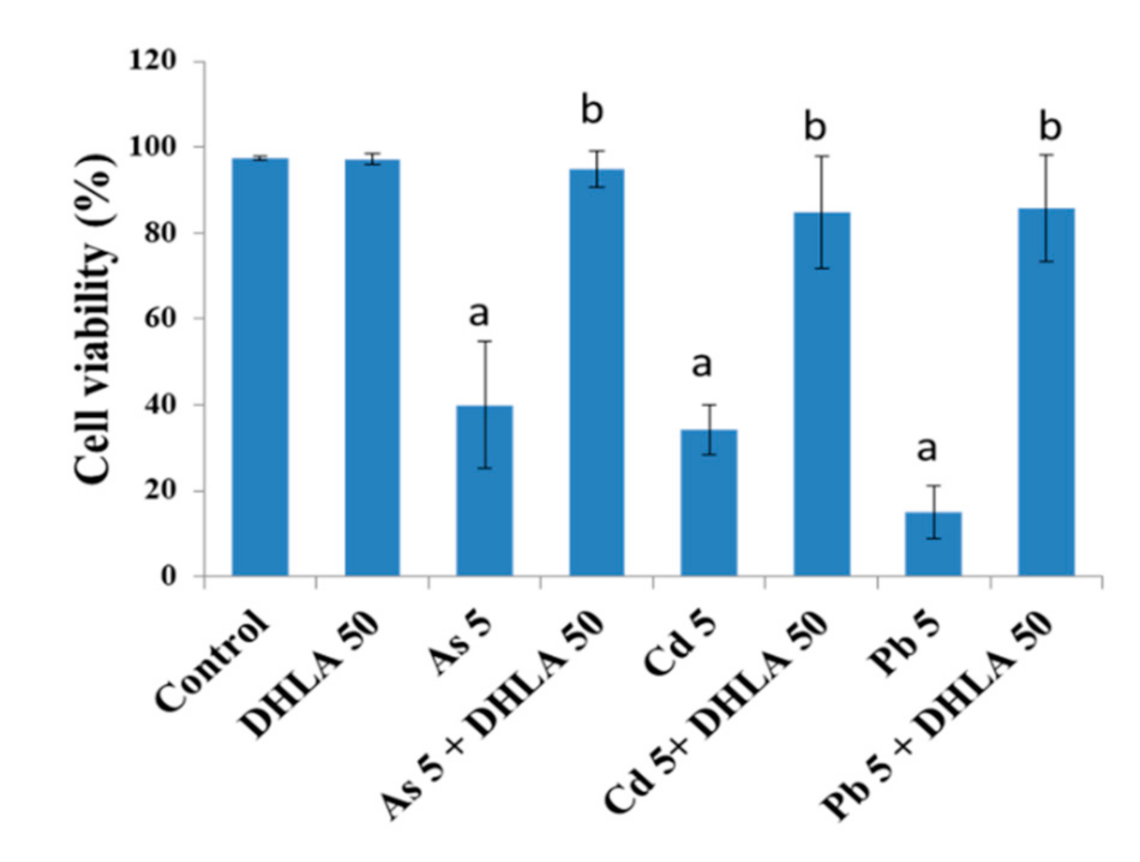
3.1. Combined Effects of Toxic Metals and ALA/DHLA on Cell Viability
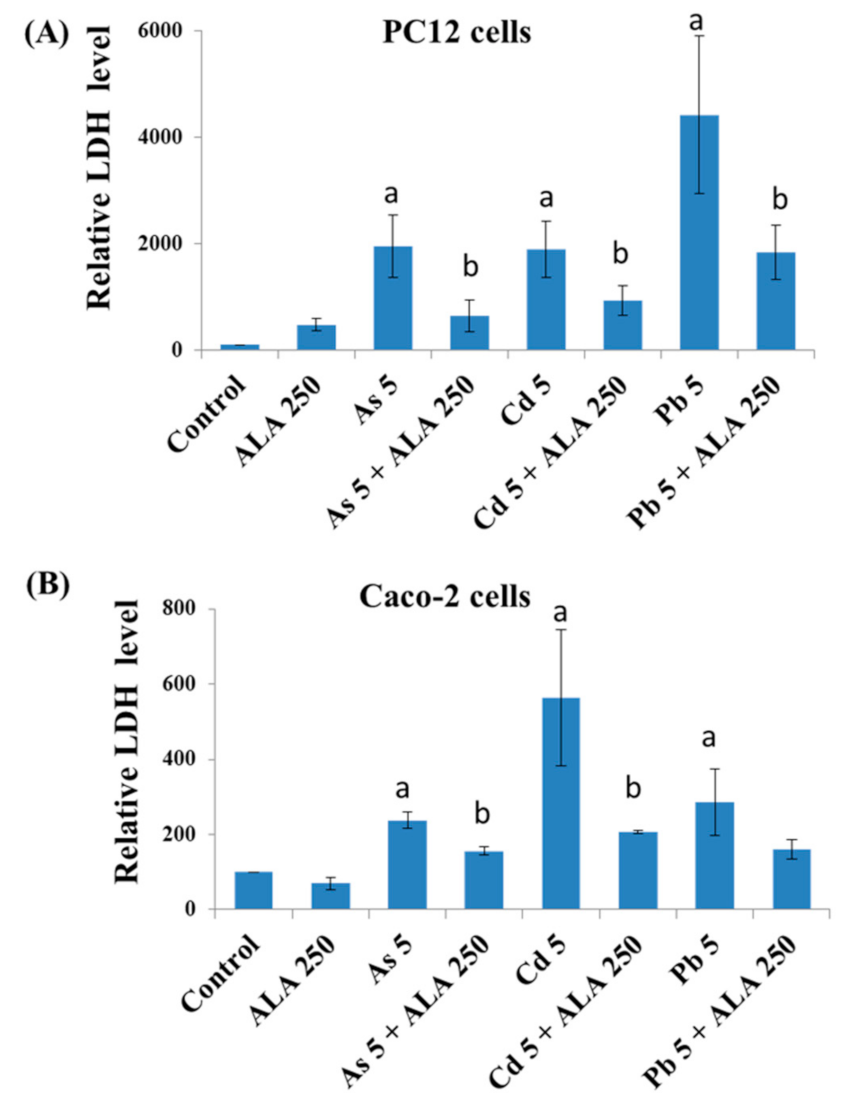
3.2. Combined Effects of Toxic Metals and ALA/DHLA on Cell Membrane Integrity
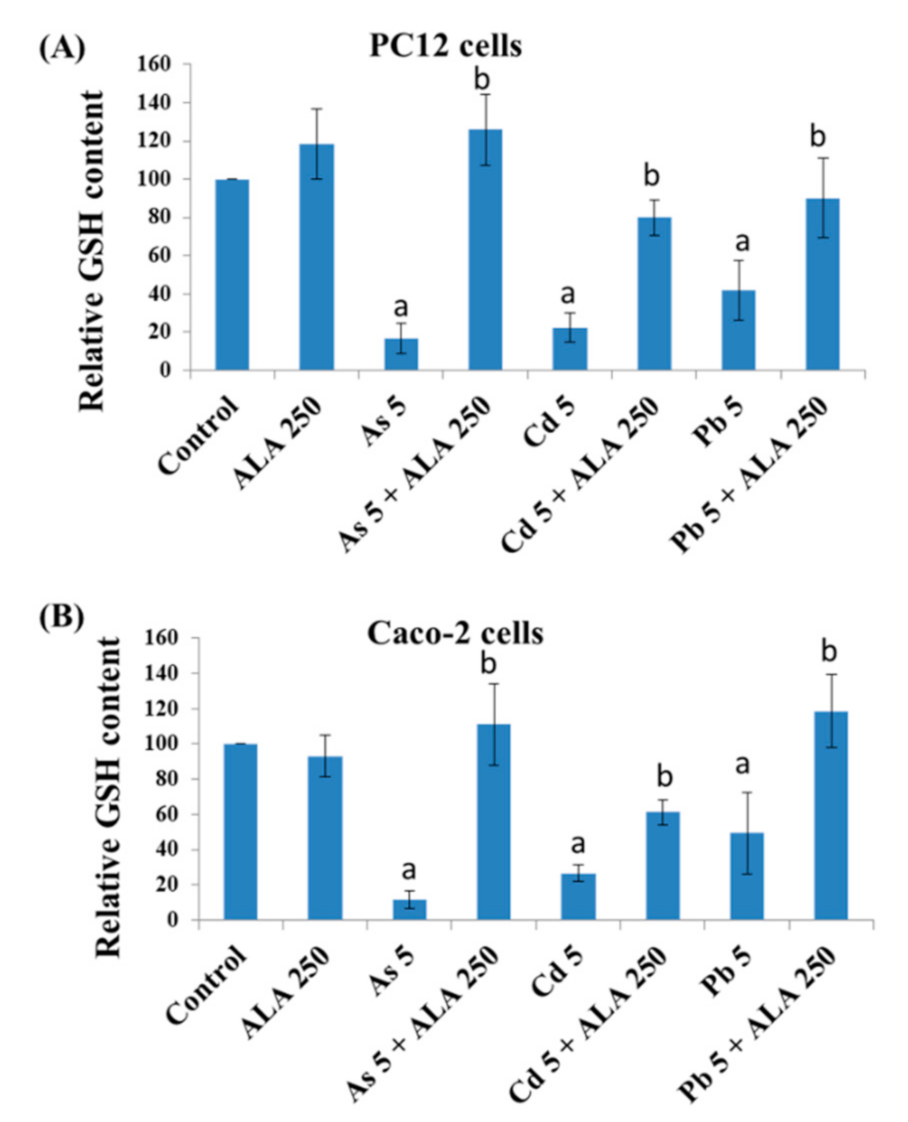
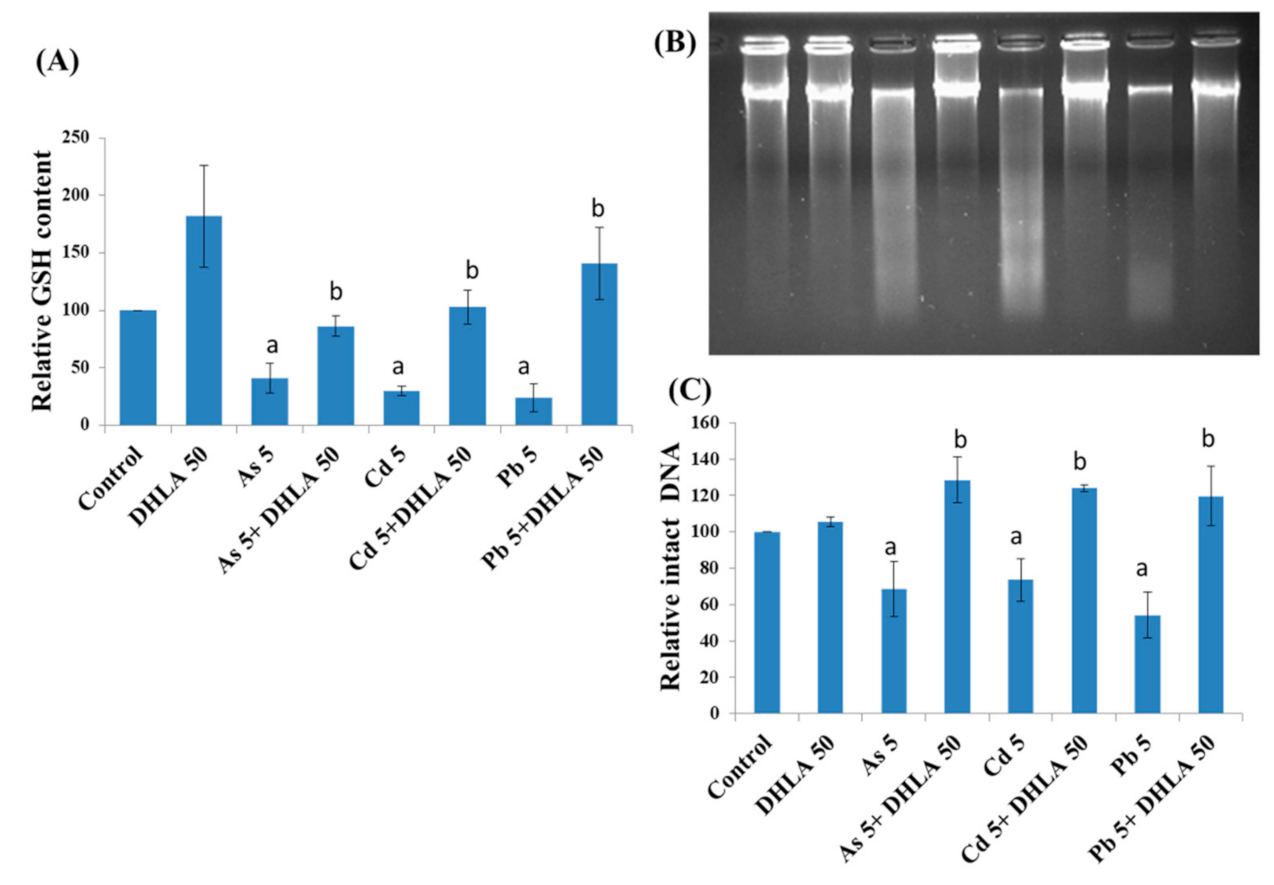
3.3. Combined Effects of Toxic Metals and ALA/DHLA on GSH Level
3.4. Combined Effects of Toxic Metals and ALA/DHLA on DNA Damage
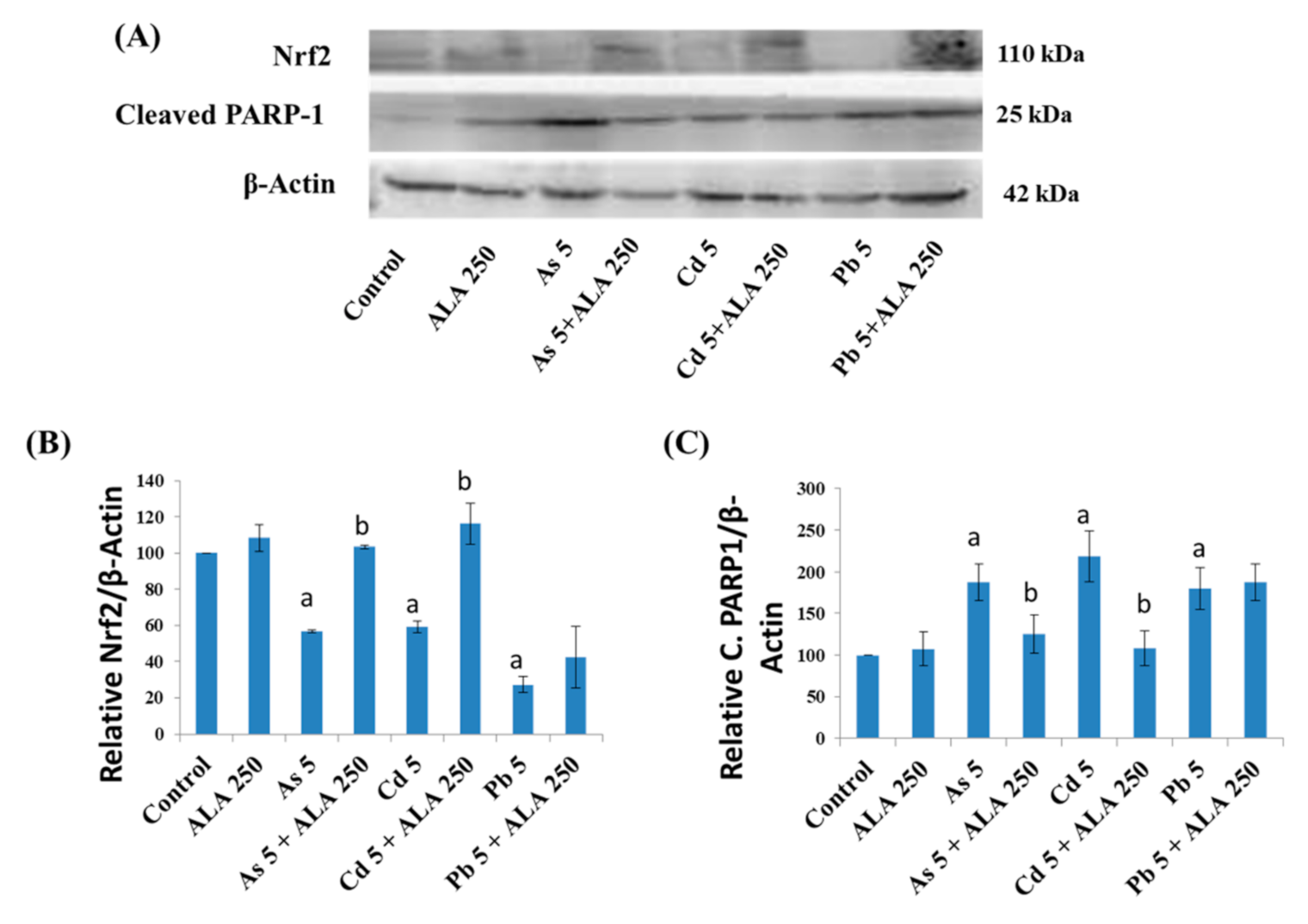
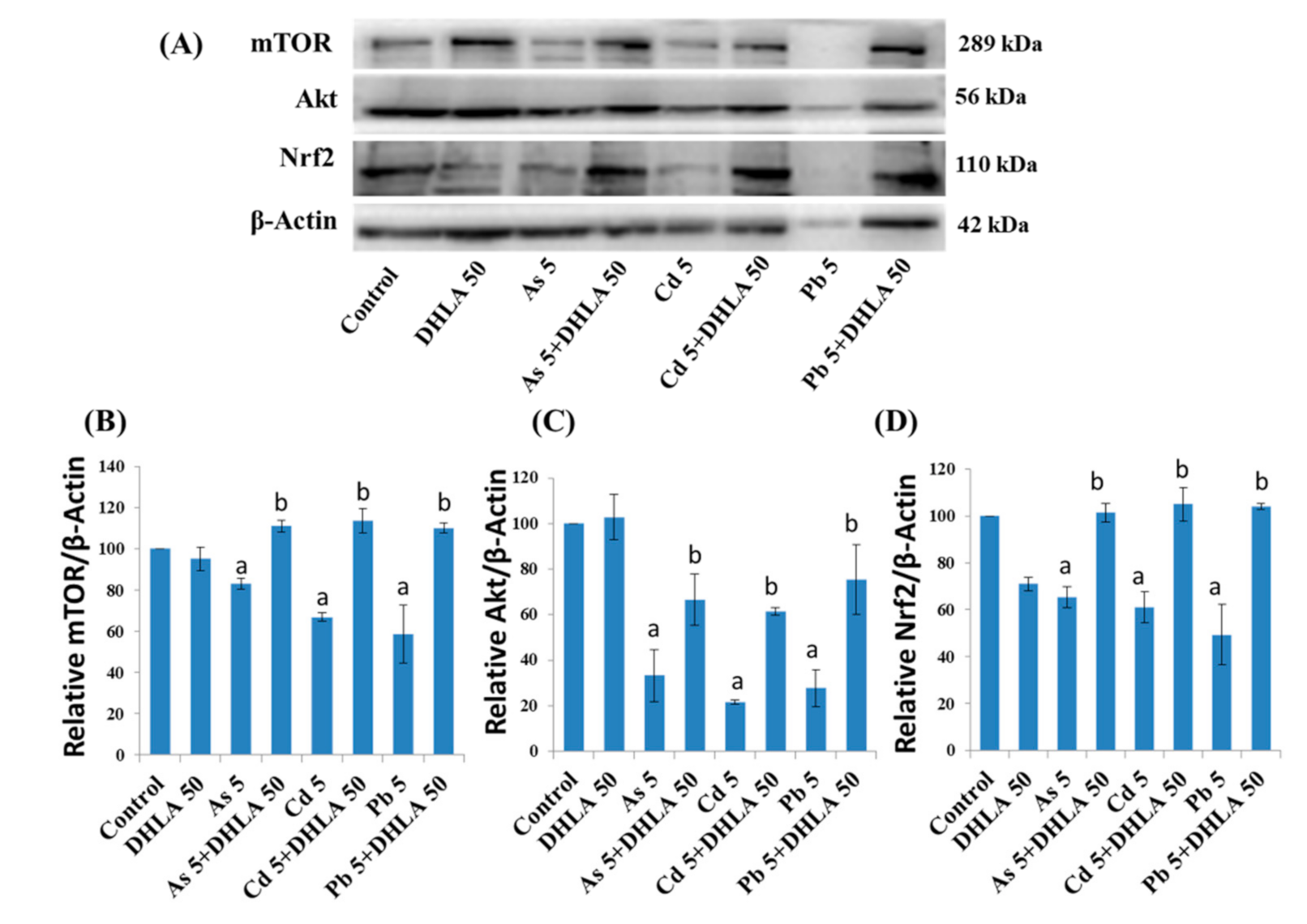
3.5. Combined Effects of Toxic Metals and ALA/DHLA on Protein Expressions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 2009, 1790, 1149–1160. [Google Scholar] [CrossRef]
- Holmquist, L.; Stuchbury, G.; Berbaum, K.; Muscat, S.; Young, S.; Hager, K.; Engel, J.; Münch, G. Lipoic acid as a novel treatment for Alzheimer’s disease and related dementias. Pharmacol. Ther. 2017, 113, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Tibullo, D.; Li Volti, G.; Giallongo, C.; Grasso, S.; Tomassoni, D.; Anfuso, C.D.; Lupo, G.; Amenta, F.; Avola, R.; Bramanti, V. Biochemical and clinical relevance of alpha lipoic acid: Antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm. Res. 2017, 66, 947–959. [Google Scholar] [CrossRef] [PubMed]
- El-Senousey, H.K.; Chen, B.; Wang, J.Y.; Atta, A.M.; Mohamed, F.R.; Nie, Q.H. Effects of dietary vitamin C, vitamin E, and alpha-lipoic acid supplementation on the antioxidant defense system and immune-related gene expression in broilers exposed to oxidative stress by dexamethasone. Poult. Sci. 2017, 97, 30–38. [Google Scholar] [CrossRef]
- Sudheesh, N.P.; Ajith, T.A.; Janardhanan, K.K.; Krishnan, C.V. Palladium-α-lipoic acid complex attenuates alloxan-induced hyperglycemia and enhances the declined blood antioxidant status in diabetic rats. J. Diabetes 2011, 3, 293–300. [Google Scholar] [CrossRef]
- Smith, A.R.; Shenvi, S.V.; Widlansky, M.; Suh, J.H.; Hagen, T.M. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr. Med. Chem. 2004, 11, 1135–1146. [Google Scholar] [CrossRef]
- Suh, J.H.; Shenvi, S.V.; Dixon, B.M.; Liu, H.; Jaiswal, A.K.; Liu, R.M.; Hagen, T.M. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. USA 2004, 101, 3381–3386. [Google Scholar] [CrossRef]
- Demir, R.; Yayla, M.; Akpinar, E.; Cakir, M.; Calikoglu, C.; Ozel, L.; Ozdemir, G.; Mercantepe, T. Protective effects of alpha-lipoic acid on experimental sciatic nerve crush injury in rats: Assessed with functional, molecular and electromicroscopic analyses. Int. J. Neurosci. 2014, 124, 935–943. [Google Scholar] [CrossRef]
- Kocaoğlu, S.; Aktaş, Ö.; Zengi, O.; Tufan, A.; KaragözGüzey, F. Effects of alpha lipoic acid on motor function and antioxidant enzyme activity of nerve tissue after sciatic nerve crush injury in rats. Turk Neurosurg. 2017. [Google Scholar] [CrossRef]
- Sanders, L.L.O.; de Souza Menezes, C.E.; Chaves Filho, A.J.M.; de Almeida Viana, G.; Fechine, F.V.; Rodrigues de Queiroz, M.G.; Gonçalvez da Cruz Fonseca, S.; Mendes Vasconcelos, S.M.; Amaral de Moraes, M.E.; Gama, C.S.; et al. α-Lipoic Acid as Adjunctive Treatment for Schizophrenia: An Open-Label Trial. J. Clin. Psychopharmacol. 2017, 37, 697–701. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ukiana, J.; Lopez, R.U.; Sikder, M.T.; Saito, T.; Kurasaki, M. Cytotoxic effects of cadmium and zinc co-exposure in PC12 cells and the underlying mechanism. Chem. Biol. Interact. 2017, 269, 41–49. [Google Scholar] [CrossRef]
- Hossain, K.F.B.; Hosokawa, T.; Saito, T.; Kurasaki, M. Amelioration of butylated hydroxytoluene against inorganic mercury induced cytotoxicity and mitochondrial apoptosis in PC12 cells via antioxidant effects. Food Chem. Toxicol. 2020, 146, 111819. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.F.B.; Hosokawa, T.; Saito, T.; Kurasaki, M. Zinc-pretreatment triggers glutathione and Nrf2-mediated protection against inorganic mercury-induced cytotoxicity and intrinsic apoptosis in PC12 cells. Ecotoxol. Environ. Saf. 2021, 207, 111320. [Google Scholar]
- Kihara, Y.; Tanaka, M.; Gumiri, S.; Ardianor Hosokawa, T.; Tanaka, S.; Saito, T.; Kurasaki, M. Mechanism of the toxicity induced by natural humic acid on human vascular endothelial cells. Environ. Toxicol. 2014, 29, 916–925. [Google Scholar] [CrossRef]
- Hossain, K.F.B.; Rahman, M.M.; Sikder, M.T.; Saito, T.; Hosokawa, T.; Kurasaki, M. Inhibitory effects of selenium on cadmium-induced cytotoxicity in PC12 cells via regulating oxidative stress and apoptosis. Food Chem. Toxicol. 2018, 114, 180–189. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lopez-Uson, A.R.; Sikder, M.T.; Tan, G.; Hosokawa, T.; Saito, T.; Kurasaki, M. Ameliorative effects of selenium on arsenic-induced cytotoxicity in PC12 cells via modulating autophagy/apoptosis. Chemosphere 2018, 196, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Arriaga, R.; Alvarez-Idaboy, J.R. Lipoic Acid and A. A Comprehensive, Theoretical Study of Their Antioxidant Activity Supported by Available Experimental Kinetic Data. J. Chem. Inf. Model. 2014, 54, 1642–1652. [Google Scholar] [CrossRef]
- Guven, A.; Tunc, T.; Topal, T.; Kul, M.; Korkmaz, A.; Gundogdu, G.; Onguru, O.; Ozturk, H. Alpha-lipoic acid and ebselen prevent ischemia/reperfusion injury in the rat intestine. Surg. Today 2008, 38, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Ghibu, S.; Richard, C.; Zeller, M.; Cottin, Y.; Vergely, C. Direct and indirect antioxidant properties of α-lipoic acid and therapeutic potential. Mol. Nutr. Food Res. 2013, 57, 114–125. [Google Scholar] [CrossRef]
- Venigalla, M.; Sonego, S.; Gyengesi, E.; Sharman, M.J.; Münch, G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2016, 95, 63–74. [Google Scholar] [CrossRef]
- Kan, E.; Alici, Ö.; Kan, E.K.; Ayar, A. Effects of alpha-lipoic acid on retinal ganglion cells, retinal thicknesses, and VEGF production in an experimental model of diabetes. Int. Ophthalmol. 2017, 37, 1269–1278. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef]
- Koloverou, E.; Tambalis, K.; Panagiotakos, D.B.; Georgousopoulou, E.; Chrysohoou, C.; Skoumas, I.; Tousoulis, D.; Stefanadis, C.; Pitsavos, C.; ATTICA study group. Moderate physical activity reduces 10-year diabetes incidence: The mediating role of oxidative stress biomarkers. Int. J. Public Health 2017, 63, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Dorsam, B.; Goder, A.; Seiwert, N.; Kaina, B.; Fahrer, J. Lipoic acid induces p53-independent cell death in colorectal cancer cells and potentiates the cytotoxicity of 5-fluorouracil. Arch. Toxicol. 2015, 89, 1829–1846. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Tate, J.J.; Cooper, T.G. Ure2, a prion precursor with homology to glutathione S-transferase, protects Saccharomyces cerevisiae cells from heavy metal ion and oxidant toxicity. J. Biol. Chem. 2003, 278, 12826–12833. [Google Scholar] [CrossRef]
- Lawal, A.O.; Ellis, E. Differential sensitivity and responsiveness of three human cell lines HepG2, 1321N1 and HEK 293 to cadmium. J. Toxicol. Sci. 2010, 35, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.F.B.; Rahman, M.M.; Sikder, M.T.; Hosokawa, T.; Saito, T.; Kurasaki, M. Regulatory effects of dihydrolipoic acid against inorganic mercury-mediated cytotoxicity and intrinsic apoptosis in PC12 cells. Ecotoxicol. Environ. Saf. 2020, 192, 110238. [Google Scholar] [CrossRef]
- Bharat, S.; Cochran, B.C.; Hsu, M.; Liu, J.; Ames, B.N.; Andersen, J.K. Pre-treatment with R-lipoic acid alleviates the effects of GSH depletion in PC12 cells: Implications for Parkinson’s disease therapy. Neurotoxicology 2002, 23, 479–486. [Google Scholar] [CrossRef]
- Jeyapaul, J.; Jaiswal, A.K. Nrf2 and c-Jun regulation of antioxidant response element (ARE)-mediated expression and induction of gamma-glutamylcysteine synthetase heavy subunit gene. Biochem. Pharmacol. 2000, 59, 1433–1439. [Google Scholar] [CrossRef]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling inoxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef]
- Wang, X.J.; Hayes, J.D.; Henderson, C.J.; Wolf, C.R. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 19589–19594. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhou, X.; Zhang, J.; Wang, J.; Xie, H.; Wu, Z. α-Lipoic acid protects against the cytotoxicity and oxidative stress induced by cadmium in HepG2 cells through regeneration of glutathione by glutathione reductase via Nrf2/ARE signaling pathway. Environ. Toxicol. Pharmacol. 2016, 45, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Macias-Barragan, J.; Huerta-Olvera, S.G.; Hernandez-Cãnaveral, I.; Pereira-Suarez, A.L.; Montoya-Buelna, M. Cadmium and α-lipoic acid activate similar de novo synthesis and recycling pathways for glutathione balance. Environ. Toxicol. Pharmacol. 2017, 52, 38–46. [Google Scholar] [CrossRef]
- Chaitanya, G.V.; Alexander, J.S.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Desnoyers, S.; Ottaviano, Y.; Davidson, N.E.; Poirier, G.G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: An early marker of chemotherapy-induced apoptosis. Cancer Res. 1993, 53, 3976–3985. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, K.F.B.; Akter, M.; Rahman, M.M.; Sikder, M.T.; Rahaman, M.S.; Yamasaki, S.; Kimura, G.; Tomihara, T.; Kurasaki, M.; Saito, T. Amelioration of Metal-Induced Cellular Stress by α-Lipoic Acid and Dihydrolipoic Acid through Antioxidative Effects in PC12 Cells and Caco-2 Cells. Int. J. Environ. Res. Public Health 2021, 18, 2126. https://doi.org/10.3390/ijerph18042126
Hossain KFB, Akter M, Rahman MM, Sikder MT, Rahaman MS, Yamasaki S, Kimura G, Tomihara T, Kurasaki M, Saito T. Amelioration of Metal-Induced Cellular Stress by α-Lipoic Acid and Dihydrolipoic Acid through Antioxidative Effects in PC12 Cells and Caco-2 Cells. International Journal of Environmental Research and Public Health. 2021; 18(4):2126. https://doi.org/10.3390/ijerph18042126
Chicago/Turabian StyleHossain, Kaniz Fatima Binte, Mahmuda Akter, Md. Mostafizur Rahman, Md. Tajuddin Sikder, Md. Shiblur Rahaman, Shojiro Yamasaki, Goh Kimura, Tomomi Tomihara, Masaaki Kurasaki, and Takeshi Saito. 2021. "Amelioration of Metal-Induced Cellular Stress by α-Lipoic Acid and Dihydrolipoic Acid through Antioxidative Effects in PC12 Cells and Caco-2 Cells" International Journal of Environmental Research and Public Health 18, no. 4: 2126. https://doi.org/10.3390/ijerph18042126
APA StyleHossain, K. F. B., Akter, M., Rahman, M. M., Sikder, M. T., Rahaman, M. S., Yamasaki, S., Kimura, G., Tomihara, T., Kurasaki, M., & Saito, T. (2021). Amelioration of Metal-Induced Cellular Stress by α-Lipoic Acid and Dihydrolipoic Acid through Antioxidative Effects in PC12 Cells and Caco-2 Cells. International Journal of Environmental Research and Public Health, 18(4), 2126. https://doi.org/10.3390/ijerph18042126









